comes from
technology
Core Value
Global phase 3 clinical development
of VVZ-149 (Opiranserin) discovered
by the ‘multi-target drug development platform’
-
Development of new chemical drugs
that create synergy by acting
on multiple targets -
It is Vivozon’s unique and essential technology
for the development of new drugs for
treating pain or central nervous system disease.ChemistryBait target approach
Platform-targeted approach
Fragment library
Expanding the chemical platform
- Efficacy-based screening for the early stage of drug development to derive candidates for successful phase 2 clinical trials
-
By discovering VVZ-149 within one year of building
a multi-target new drug development platform,
it proved the effectiveness of saving time
and cost in the new drug discovery stage.BiologyTissue-level efficacy assays
Optimized in vivo screening
ADME Lab leading to the initial development
Establishment of independent/in-house in vitro screening system
-
Securing and developing
global clinical development capabilities -
Clinical strategies for rapid development
with US FDA fast track designationClinicalTarget the global market from the beginning
In-house preparation of high-level clinical technical documentation
Global-level clinical management capabilities
US FDA fast track designation
Vivozon’s core technology
Multi-Target Therapeutics
At the end of the 20th century, with the help of advances in genetic engineering and molecular biology, the current single-target drug development paradigm was established to treat diseases by modulating the function of individual genes or single proteins. However, advances in biology have shown that a disease or function is the result of a complex interaction of multiple targets (receptors or proteins in the body). Therefore, new disciplines such as polypharmacology, network pharmacology, and multi-target therapeutics have emerged and led to disease treatments targeting multiple receptors or proteins simultaneously.
Nevertheless, numerous global pharmaceutical companies have attempted to make new analgesic drugs through a single-target approach to disease treatment due to limited practical implementation that enables a multi-target approach. Clinical trials confirmed a lack of efficacy and declared failure of the current methodology which relies on selectivity and potency for a single target for treating central nervous system diseases including pain.
Vivozon clearly recognized that the fundamental of successful strategies for drug development was therapeutic efficacy, not reactivity with the target (potency or affinity). Therefore, a phenotypic screening system was established to search for substances based on efficacy from the early development stage. By using this technology, we devised a multi-target new drug discovery
platform with use of a bait-target approach that searches for a multi-target combination amplifying therapeutic efficacy via potential synergistic or additive effects.
Currently, Vivozon has developed and is continuing to develop a multi-target new drug with clinical efficacy by aiming at two single-targets simultaneously and confirming their synergistic activities.
Bait-Target Approach
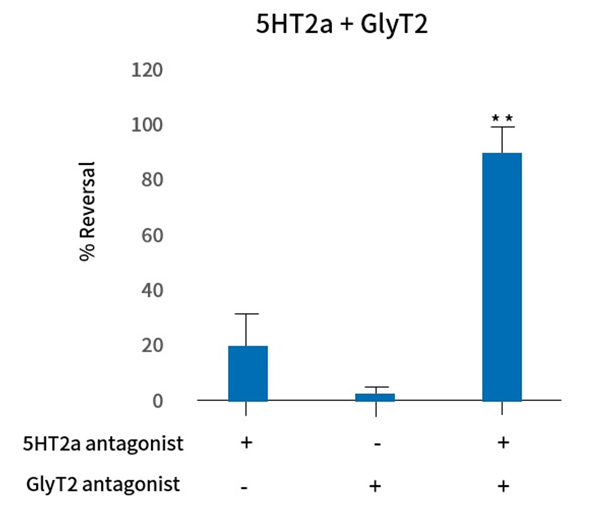
The bait-target approach is a method of finding multi-target combinations by first selecting a bait target and then finding a target that yields the greatest synergistic effect with the bait target. Vivozon has secured substances that block GlyT2, which promotes the transmission of pain from the peripheral to the central nervous system. Among them, VVZ-149, which is optimized to simultaneously inhibit 5HT2a involved in pain signal transmission, was selected as the final substance and developed as a lead pipeline. If the clinical development of VVZ-149 is successful, the validity and utility of Vivozon’s technology will be proven. We expect to provide a new multi-target drug discovery methodology globally.
Platform-Target Approach
5HT2a is a relatively safe protein related to a wide range of the central nervous system (CNS) physiology and is known as a very suitable target for the development of new CNS drugs. Currently, Vivozon is discovering new substances that can produce multi-target analgesic effects by identifying candidates applicable to 5HT2a and then characterizes potential effects on another target.
In particular, 5HT2a has been confirmed its greater analgesic efficacy as a multi-target rather than a single target. Through this platform-target approach, Vivozon has derived new substances such as VVZ-2471 and will continue to establish a long-term pipeline.
Phenotypic Screening
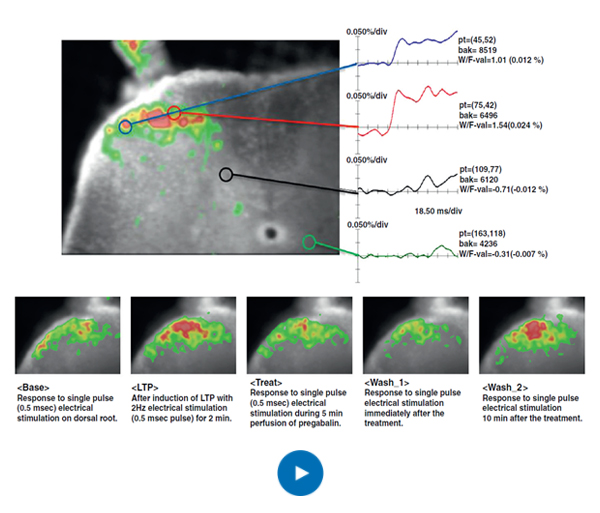
Vivozon selects candidates through phenotypic screening to improve clinical success. This is a method of monitoring the neural activity of tissues related to pain while culturing tissues collected from living animals. This phenotypic screening method using electrophysiological techniques at the tissue level (ex vivo) has the advantage of being able to confirm the physiological efficacy of a candidate material more reliably than the commonly used target protein or cell level screening method. When this method is combined with an in vivo model that targets an organism in a living state, it can more reliably support the prediction of clinical efficacy. These techniques can be extended to various diseases as well as pain.
PK/PD correlation and blind test
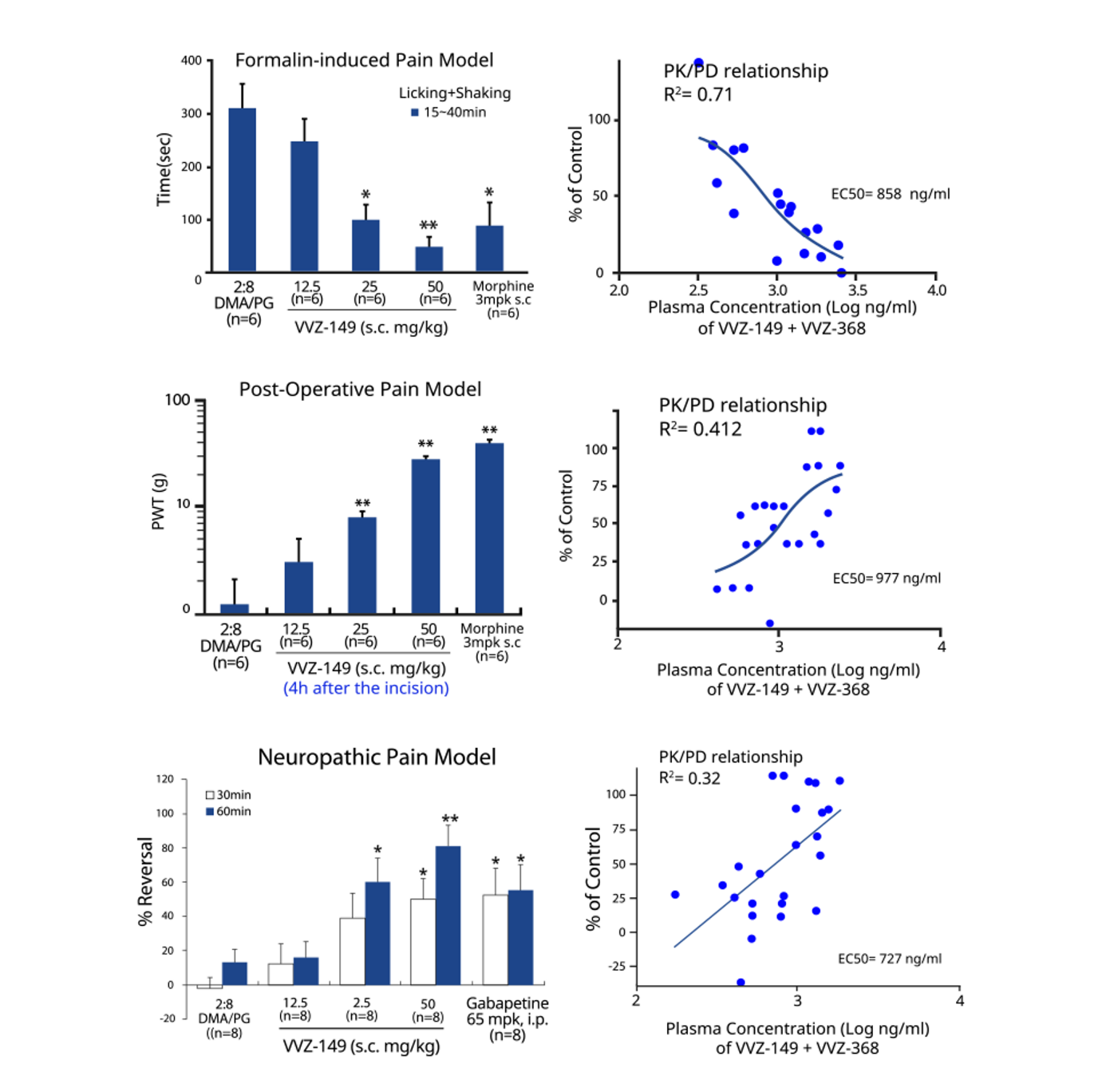
The final gateway for new drug candidates toward clinical trials is in vivo efficacy testing on animals. A blind test involves observing and measuring pain without knowing which drug has been administered to the animal. This is essential to rule out any bias or subjectivity on the part of the researcher/experimenter and to maintain objectivity, which is the fundamental purpose of science.
In addition, the efficacy of the test drug should be checked for a meaningful correlation between the blood concentration and efficacy of the animal, as in clinical trials, after drug administration from a pharmacological point of view.
Vivozon has the in vivo testing technology with global competence and validates animal efficacy on the basis of basic principles including blinding.